#UX
#IxD
#web
#Figma
TrialAI
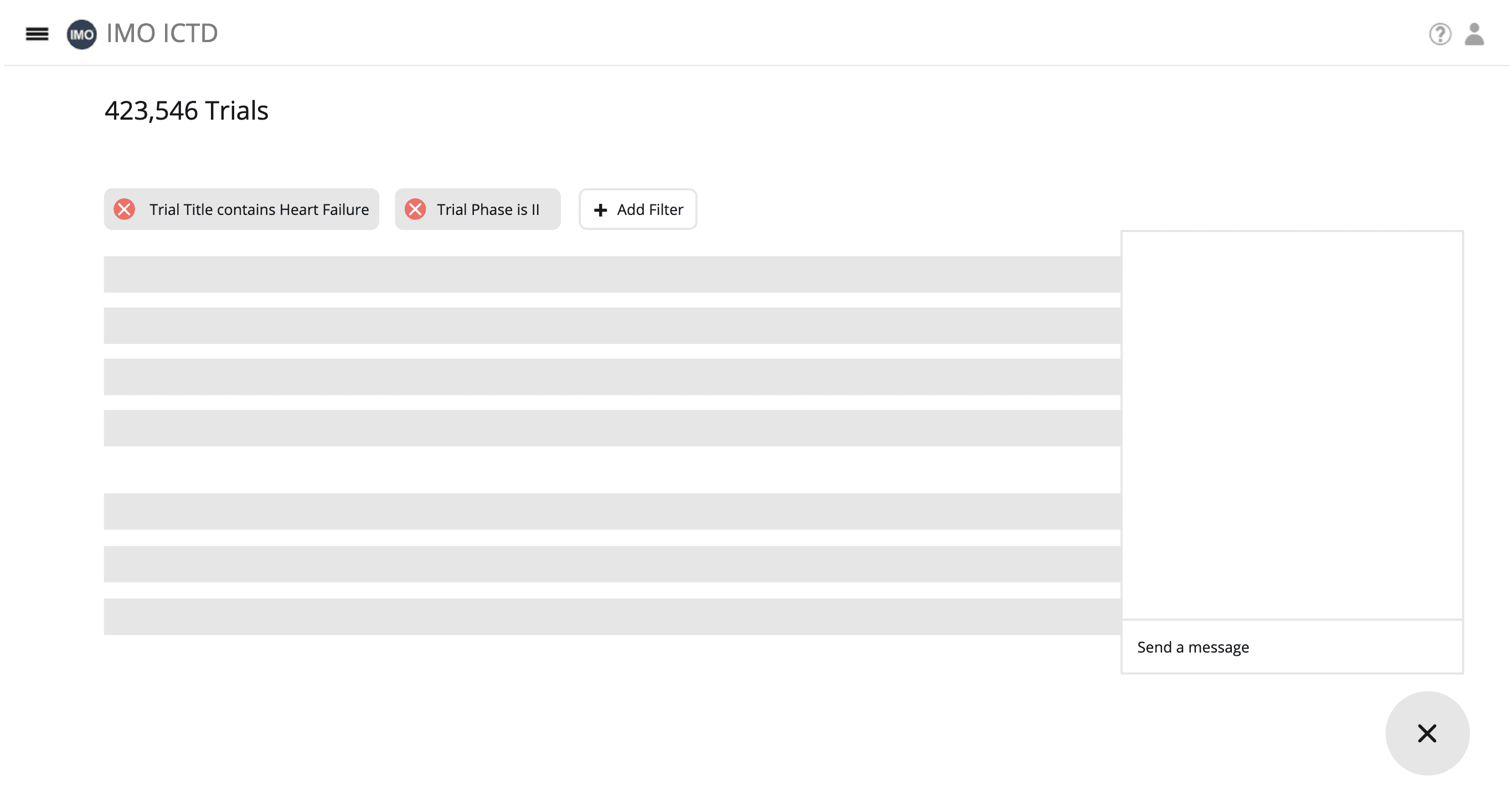
This application will provide users with a set of functionalities to search, visualize, analyze, and generate inclusion and exclusion criteria for clinical trials. The primary goal is to enhance the efficiency and accuracy of clinical trial design for those with clinical trial knowledge.
Timeline
Role
Why is the world broken?
The average cost of phase 1, 2, and 3 clinical trials across therapeutic areas is around $4, 13, and 20 million respectively. If we can introduce the utilization of the Large Language Model, it could not only make the process more efficient, but also more accurate.
The user

Name: Dr. Sarah Thompson
Age: 37
Gender: Female
Education: Ph.D. in Clinical Research
Occupation: Principal Investigator at a renowned research institution.
Background: Sarah is in her late 30s, an age where she has established herself in her career and is seeking to make significant contributions to her field. As a principal investigator, she holds a position of authority and responsibility within her research institution. Her academic background reflects a deep understanding of clinical research methodologies and a commitment to advancing scientific knowledge.
Actions & Motivations
1
What do I do?
Dr. Thompson oversees the design and implementation of clinical trials in the oncology department.
She collaborates with a multidisciplinary team of researchers, data analysts, and statisticians.
Dr. Thompson is responsible for ensuring that her trials comply with regulatory standards and ethical guidelines.
2
Why do I do it?
She's driven by a passion for contributing to scientific and medical advancements.
She is also motivated to improving patient outcomes by conducting impactful clinical trials.
She values collaboration and seeks tools that enhance teamwork and communication within her research team.
3
What do I want?
Being able to design and conduct innovative clinical trials that contribute to advancements in cancer treatment.
Staying up-to-date with the latest research methodologies, regulatory requirements, and technological advancements in clinical trial design.
Collaborating efficiently with her team to streamline the trial design process and improve overall research productivity.
Pains
1
What's stopping me?
Balancing the need for comprehensive inclusion/exclusion criteria with the demand for quick and efficient trial design.
Managing a heavy workload, including protocol development, grant writing, and mentoring junior researchers.
Ensuring seamless communication and collaboration among team members, especially when working on multiple trials simultaneously.
How do we fix it?
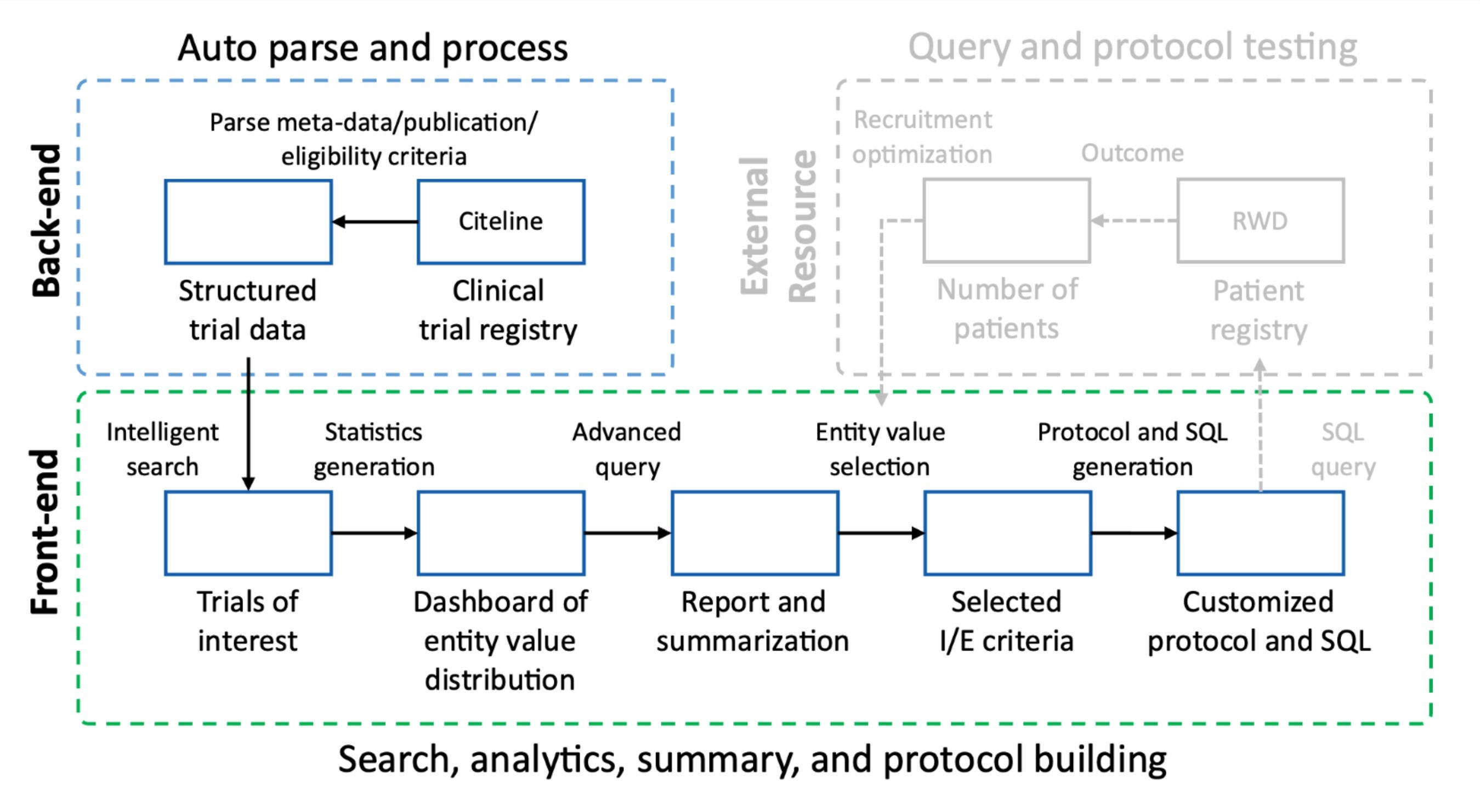
Intelligent Search: Users can perform Question & keyword-based searches on preprocessed Clinicaltrials.gov / Citeline data.
Data Aggregation(dashboard): The application will combine and present the results from question & keyword-based searches. ( hist / bar chat ).
Inclusion and Exclusion Criteria Generation: The application will generate an initial version of inclusion and exclusion criteria based on the obtained data.
Question and Answer: Users can ask questions about the clinical trial data and receive relevant answers.
Adjustment of Criteria: Users can adjust the generated inclusion and exclusion criteria directly and by providing English instructions.
Lo-Fi
The main interaction point was always between the chat functionality and how that worked with the dashboard view. We landed on a sidebar treatment to allow the user to expand/collaps the chat window at any point in the flow.
Prototype
We provided a high-fidelity prototype to share with potential users within the field. Development is slated to complete in Q2 of 2024. See it here
Metrics
Coming soon - in development
Tools
Figma
Conclusion
Large language models like ChatGPT enhance clinical trial design by analyzing vast literature, optimizing protocols, and predicting outcomes based on existing data. Additionally, these models aid in risk assessment, ethical compliance, and regulatory adherence, ensuring trials uphold standards and participant safety. Through real-time monitoring and adaptive design, they enable researchers to adjust protocols and interventions for improved outcomes. Overall, large language models serve as valuable decision support tools, offering insights and recommendations to enhance the efficiency and effectiveness of clinical trials.